- Have any question?
- 02 7200 2179
- media@healthcarechannel.co
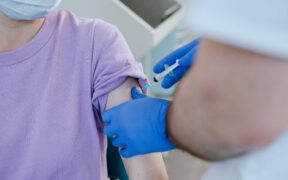
Novavax COVID-19 vaccine (Nuvaxovid) has been provisionally approved by the Australian Therapeutic Goods Agency (TGA) for use in adolescents aged 12 to 17 years. The
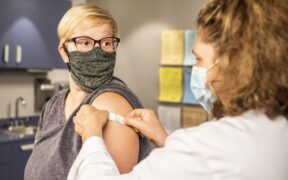
Novavax has been approved as a COVID-19 booster for people aged 18 and above. In a statement released on Tuesday, Novavax announced the Therapeutic Goods Administration had

The Australian Technical Advisory Group on Immunisation (ATAGI) has recommended the use of the Novavax vaccine as a booster in Australians aged 18 and over
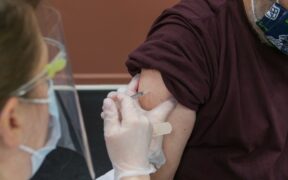
The first shipment of the Novavax COVID vaccine arrived in Australia last night ahead of the rollout later this month. The shipment, containing about 3 million
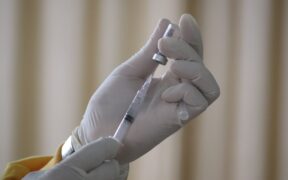
Australians aged 18+ will now have access to a fourth COVID-19 vaccine, Nuvaxovid (Novavax) in coming weeks, after the Australian Government accepted advice from the

Australians who are severely immunocompromised will be offered the option to receive a third COVID-19 vaccine dose to boost their protection against COVID-19 to the